COVID-19 PCR Test
Accurate MedLab, LLC offers COVID-19 real time PCR testing. Our SARS-CoV-2 detection is a real-time reverse transcription polymerase chain reaction (RT-PCR) assay. Our PCR test and results are appropriate and accepted for international travel and returning to work/school.
Our PCR test is approved for emergency use by the FDA. Click here to view FDA, Emergency Use Authorization (EUA).
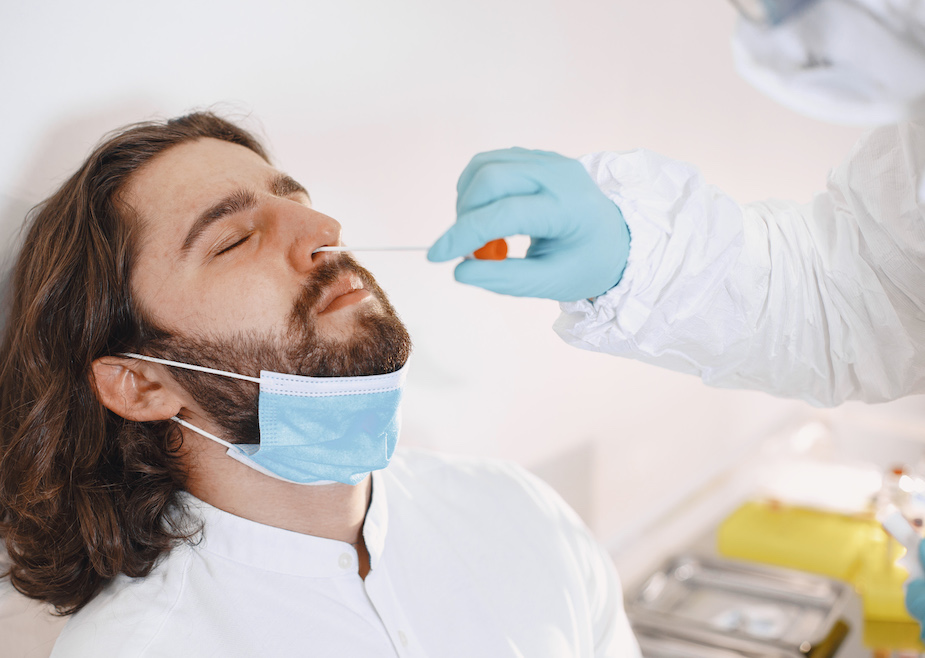
Collection Kits
Yes, Accurate Medlab can provide you with COVID-19 collection kits. Our collection kits are suitable for nasopharyngeal and oropharyngeal collections.
For Providers

Accurate Medlab is a CLIA certified, high complexity laboratory, and has been in business for years. We currently provide COVID-19 RT-PCR testing for many local medical providers.
As a provider, you can place orders and view results online, or we can provide integration with most EMRs. Our local drivers would be happy to pickup patient specimens, or drop them off at our laboratory near the Buffalo airport. Results are typically available the next day; no laboratory is returning results faster.
Please give us a call for more information 630-317-7645
Results
Patients can view results right here on our website. Results are typically available in 24 – 48 hours.
Main Office – 246 E. Janata Blvd Suite 110 Lombard, IL 60148 (view map)
Valid photo ID is required to pick up results in-person.

Providers can use our portal to receive and view patient results. Simply follow the Portal Login link in our menu above. We also integrate with most EMRs. Please contact Accurate Medlab if you are interested in working with Accurate Medlab for your testing needs.

CPT Codes
U0003 - $150.00 - COVID 19 PCR Test
(Infectious agent detection by nucleic acid (DNA or RNA); severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [COVID-19]), 2 amplified probe technique, making use of high throughput technologies as described by CMS-2020-01-R)
U0005 - $50.00 - COVID 19 Expedited Results within 48 Hours
(Infectious agent detection by nucleic acid (DNA or RNA); severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [COVID-19]), amplified probe technique, CDC or non-CDC, making use of high throughput technologies, completed within two calendar days from date and time of specimen collection. (List separately in addition to either HCPCS code U0003 or U0004)
87811 - $100.00 - Rapid Covid Antigen Test
(Infectious agent antigen detection by immunoassay with direct optical (ie, visual) observation; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [COVID-19])
G2023 - $50.00 - Collection Fee of Specimen
(Specimen collection for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Coronavirus disease [COVID-19]), any specimen)

